In this time of different coatings, materials and manufacturing processes, what are the factors important to surgeons when choosing an interbody fusion device?
Early in 2018, MedTech Strategies conducted a brief online survey with 24 US based orthopedic surgeons to collect input on this question. Surgeons were asked to rank the importance of several factors and key aspects of visualization that may be considered when choosing interbody fusion devices. Specifically, how important was their ability to:
• Visualize and position the device intraoperatively?
• Visualize the graft material in and around the device intraoperatively?
• See a ‘good fit’ and endplate contact?
• Visualize solid bone in the center of the device?
• Visualize continuous bridging bone to verify fusion?
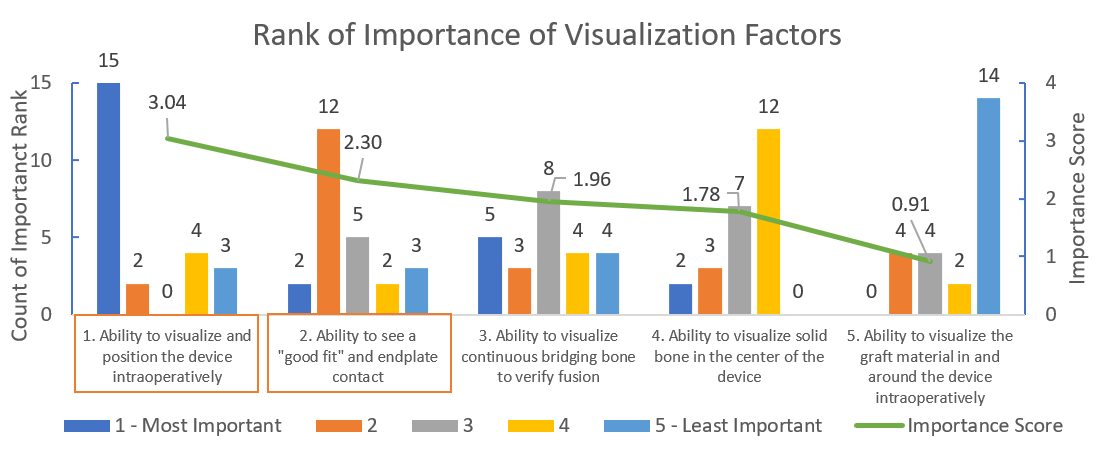
MedTech Strategies found that two factors emerged above the others. The “Ability to visualize and position the device intraoperatively” and the “Ability to see a ‘good fit’ and endplate contact” were ranked the highest and may therefore be most important to take into consideration when designing and choosing appropriate interbody fusion devices. See Figure 1.
In performing this ranked assessment of factors impacting visualization, a significant difference in rankings (p<0.001) as evaluated by the Friedman Test was found.
Figure 1: Rank of importance of visualization factors
Spine companies are looking for improved ways to maximize bony integration and fusion rates. Changes in manufacturing approaches, such as titanim coatings, refined compositional materials and surface designs, are contributing to improvements in offerings. In particlar, 3D printed devices are being desgined to improve bony ongrowth or ingrowth without sacraficing strength or modulus of elasticity.
In this study, surgeons were presented with 4 commercially available 3D printed devices via the following radiograph images and, reflecting on their need for visualization, asked to choose their preferred device based on the following images. See Figure 2.
• Stryker Tritanium®
• Titan ENDOSKELETON®
• HD LifeSciences Hive™
• 4Web Truss System™
Figure 2: Radiographic images from tested devices
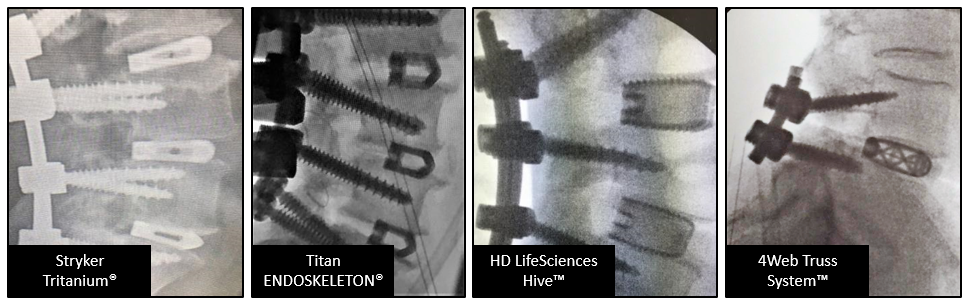
HD LifeSciences was chosen as the most preferred receiving 82% of all possible Preference Points, followed by Titan (71%), 4Web (38%) and lastly Stryker who received only 10% of possible Preference Points. (Preference Points were awarded to a device for each ranking received of 1-4. The resulting points were converted to a percentage, compared to the other devices and analyzed via statistical test to confirm rank (p<0.001).)
Manufacturers of the new generation of interbody devices must consider a variety of factors when looking to break into the crowded interbody fusion device market. Visualization is a key element that must be weighed amongst the other factors potentially leading to device, and therefore surgical, success. In the visualization arena, HD LifeSciences has found a way to offer a new type of 3D printed device that provides the visualization demanded from today’s spine surgeons.
Ilsa Webeck is the founder and lead principal consultant of MedTech Strategies LLC, a boutique strategic marketing firm focused on understanding customer needs to build for growth. In her 20+ years in healthcare business strategy she has contributed to the success of emerging ventures and market leaders by uncovering value for new technologies. Ilsa holds a BA in Biology from Dartmouth College and an MBA from the Tuck School of Business. She is an invited lecturer at the Boston Biomedical Innovation Center, Harvard University Office of Technology Development and mentor for MassChallenge.


