Symptomatic lumbar degenerative disc disease (DDD) is a major cause of back pain, missed work days, and healthcare utilization.1; 2 If left untreated, it can lead to bone spurs, spinal canal compression, debilitating pain, herniated discs, and loss of bladder control.3; 4
Until recently, no studies investigated the long-term effectiveness and safety of Aesculap’s activL lumbar TDR. To learn more about the long-term effectiveness and safety of activL, Becker’s Spine Review recently reviewed the final longterm results from a randomized controlled FDA investigation device exemption study of the activL lumbar TDR.
Motion preservation is critical for long-term lumbar DDD management
Traditional surgical approaches to treat lumbar DDD typically involve lumbar fusion, which aims to prevent motion in order to alleviate pain caused by DDD. However, restricted motion and flexibility in the spine often places stress on neighboring segments that can lead to further degeneration, dysfunction, pain at an adjacent segment, and the need for a second surgery over the long term.5-10
As a result, lumbar TDR aims to relieve back pain while maintain more normal motion. Several robust clinical studies show equivalent or improved results compared to lumbar fusion over several outcomes, including pain, disability, range of motion, and reoperation.11-15 Lumbar TDR has also been shown to be superior to nonsurgical treatment over long-term follow-up.16-18
Final long-term study results highlight the effectiveness of lumbar TDR and activL Artificial Disc
The activL Artificial Disc is the latest-generation TDR device from Aesculap that aims to more closely align with the natural motion of the health human spine, potentially further reducing wear on facet joints and adjacent segments. Success of activL was first demonstrated in a 2-year randomized, investigation device exemption trial study that supported its non-inferiority to the lumbar TDR devices prodisc L and Charité. 19 Results compared to prodisc L were maintained through to 5-year follow-up.20
In June 2021, Aesculap published the results of their final long-term investigation device exemption study that looked at the safety and effectiveness of activL compared to prodisc L over 7 years in patients with lumbar degenerative disc disease who had failed at least 6 months of nonsurgical therapy. 21 Overall, the study demonstrated activL remained non-inferior to prodisc L at 7-year follow-up. In pre-specified secondary analyses, activL was superior for range of motion and freedom from serious adverse events over 7-year follow-up.
- Treatment Success (composite of Oswestry Disability Index [ODI], neurological status, range of motion [ROM], freedom from secondary surgery, and serious device-related adverse event): activL had similar rates of treatment success as prodisc L at seven-year follow-up (30.7% vs. 29.2%; p for noninferiority = 0.0369).
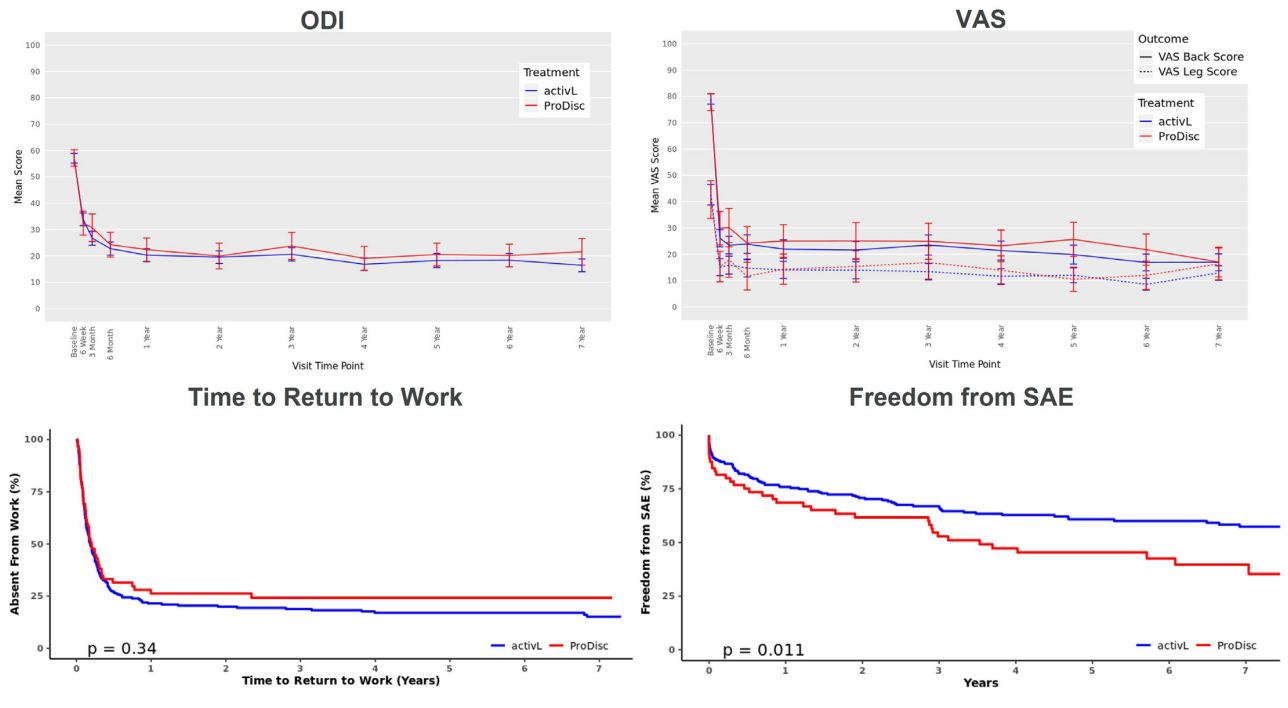
- ODI: activL showed significant reduction in ODI scores significantly decreased from baseline to 7-year follow[1]up (57 to 16; p < 0.0001) that were not statistically significantly different from prodisc L (59 to 22; p < 0.0001 for change from baseline) (Figure 1)
- Back/Leg Pain Severity Visual Analogue Scale (VAS): activL showed significant decrease in the mean VAS back and leg pain scores from baseline to 7 years (back score: 79 mm to 17mm; leg score: 43 mm to 13 mm; both p < 0.0001) that was similar to the significant decrease associated with prodisc L (back score: 78 mm to 17; leg score: 41 mm to 16 mm; both p < 0.0001) (Figure 1).
- SF-36 (Thirty-six-item Short Form Survey): The mean SF-36 Physical Component Summary (PCS) score similarly improved for both activL (by 13.1 points) and prodisc L (by 11.4 points; p < 0.0001). The mean Mental Component Summary (MCS) score also improved for activL (by 17.2 points) and prodisc L (by 18.3 points; p < 0.0001).
- Patient Willingness to have Surgery Again: More activL patients were willing to have surgery again than prodisc L patients, although this difference was not statistically significant (97.2% vs. 91.9%; p = 0.0749)
- Patient Satisfaction: Overall patient satisfaction was similar among activL and prodisc L patients (95.3% vs. 93.5%; p = 0.526
- Return to Work: activL had a shorter median return-to-work time than prodisc L (67 days vs. 74 days) that was not statistically significant (Figure 1).
- Opioid Use: Opioid use significantly decreased over 7 years with both activL (64.7% to 0%; p < 0.01) and prodisc L (63.1% to 0%; p < 0.01. No patients were using opioids after six years.
- Reoperation Rate: Reoperation rates with activL and prodisc L similarly were low over 7 years (p = 0.34 between groups). Over 95% (270/283) of activL and prodisc L patients were free from reoperations.
- ROM: activL had significantly greater mean flexion-extension rotation than prodisc L (5.3 vs. 4.1; p = 0.0334).
- Serious Adverse Events (SAEs): activL had significantly greater freedom from SAE than prodisc L (61.5% vs. 43.1%; p = 0.011) (Figure 1).
Figure 1: ODI, VAS, Time to Return to Work, and Freedom from SAE from baseline to 7 years for activL and prodisc L
Conclusion
Symptomatic lumbar DDD is becoming more common as the general population ages. Approximately 6.8% of the North American population are diagnosed with lumbar DDD yearly.22 Lumbar TDR is an effective way to treat lumbar DDD that preserves motion while shortening recovery and rehabilitation time.
Lumbar TDR shows early and significant improvement in pain and impairment following recovery from surgery, and statistically significant improvements are maintained through 7-year follow-up. activL is more effective at preserving motion and has more favorable safety profile than prodisc L.


