This article is a portion of a book titled "Challenges, Risks and Opportunities in Today's Spine World " edited by Stephen Hochschuler, MD, Frank Phillips, MD, and Richard Fessler, MD. You can find links to the previous chapters at the end of this article.
It was a warm, May afternoon in San Diego when we were finalizing documents for the sale of SeaSpine, a biotechnology corporation that I founded eleven years prior.
The price tag of $89 million plus royalties for life was to include all the products I developed over the years for use in spine surgery. It was a tough decision to let go because naturally, I had my qualms about selling a company that I started from scratch and guided through each year of its life, much like a child. We were generating over $50 million annually in sales and the relationships I had fostered with our physicians over the years was an added bonus. SeaSpine had become an extension of our family. Every tool launched, every conference held and every surgery integrating our products was a result of tremendous labor, sacrifice and passion for the field of medicine. The journey of taking it from square one to selling it to a $4 billion publicly traded company was an educational and ambitious one that shifted the paradigm in some standard spinal tools.
The way I see it, our job as physicians does not stop at science. If I can also be a toolmaker and design medical products to serve those in deep need, then I will make it my side job. If I can be an entrepreneur and mass produce the tools to help thousands of people across the country with deformities, broken backs or severe pain that debilitates their lives, then that too will be one of my tasks. What some may perceive as an insurmountable obstacle, I take as an opportunity for innovation and advancement.
The idea to innovate began swarming in my mind as early as my time in spine fellowship. Every time I picked up the scalpel and operated on a patient during my 80-hour work weeks, a vision of doing it differently—smarter, faster, more effectively—infiltrated my mind.
It was 1989 and I was training to become a spine surgeon at the University of Colorado in Denver. I had just completed my residency in orthopedic surgery at the same institution, which was much more clinically oriented, with a larger focus on patient care over research.
Dr. Anthony Dwyer and Dr. Thomas Lowe were my fellowship directors. Dr. Lowe, who has since passed away, was a reputable and busy scoliosis surgeon. Patients from neighboring states traveled to him for their surgeries. Dr. Dwyer was well known for anterior lumbar surgery with vast experience from his years in Hong Kong working on patients with Pott’s Disease. My surgical skills flourished in the program because the directors offered me vast hands-on experience and allowed me work on and solve complex cases on my own.
Although this was the final step in my academic career, for me, the work and education did not end there. It was only the beginning. My fellowship directors, trusting my surgical talent, handed me significant autonomy in signing up patients and performing solo cases. I constantly saw room for improvement—both in surgical technique and in hospital practice.
One of the first things I did was propose that we form our own back clinic at Denver General, which was the county hospital run by the university. I spearheaded the clinic’s development, which had two critical byproducts: It alleviated the shortage in spine care and had generated high revenue from the steady and continuous increase in spine surgery cases. Prior to that, all the spine cases were sent out to another local hospital—St. Anthony’s.
Under the supervision of Dr. Dwyer, I regularly scheduled a high number of cases each week and the doctors permitted me to perform the cases from start to finish. In the entire orthopedics department at Denver General spine soon became the number one producer of surgeries.
Soon after, I was exposed to the backend of surgery when I worked on crafting a rod bender. Orthopedic surgeon Dr. Marc Asher of Kansas had developed the Isola Spine Implant System—a system of screws and rods for scoliosis and lower back fusion that was used by physicians worldwide. That same year, he set out to develop a rod bending system, and sought assistance from Dr. Lowe, who delegated the job to me. I was a surgeon with no engineering background, but had enough spine training to know what to construct.
Dr. Asher had sent an engineer from AcroMed, later acquired by Johnson & Johnson’s DePuy, who I worked with to come up with the unique bender that would also allow for a deep, Galveston bend. The final product involved a long construct that went into the iliac wings, and it significantly simplified scoliosis surgeries for paralytic patients.
It was at that point that I decided to not solely practice medicine by the book, but to allow myself to problem solve untouched areas. At first it may help one patient, cut surgery time and simplify technique. Then, further research and development helps more patients and eventually advances surgery in that field, ultimately changing the face of disease management and medicine.
I initiated discussions of creating a polyaxial screw with the same toolmaker from AcroMed, but was near completing my fellowship and had plans to move my family to California. I began practicing at Community Orthopedics in Riverside, California and was already a seasoned spine guy due to my vast experience at Denver General. I collaborated with AcroMed—which by then had become the second leading spinal implant company—to work on some designs of instruments that would make surgeries safer for my patients. They provided the same type of implants I was accustomed to working with toward the end of my fellowship.
My first goal was to create a simpler and safer method for removing spinal discs from between the vertebral bodies in anterior lumbar fusions. The existing, widespread approach involved use of a knife to excise the disc. This carried great risk and posed complications for unskilled surgeons and those who haphazardly rush through cases. Unfortunately, surgeons are not infallible and some can cut too deep, permanently injuring nerves and vessels. I set out to design a shaver with a specific depth and variable heights to allow for a soft shave, rather than a sharp poke to the tender area. The flexibility of adjusting the height of each shaver allowed safe removal of the disc without damaging the vertebral body/bodies. It decreased surgery time and more importantly provided an unmatched level of safety. With more safety comes better results and with that, more hope for tackling the epidemic of back pain.
Image 1: Sketch of the shaver
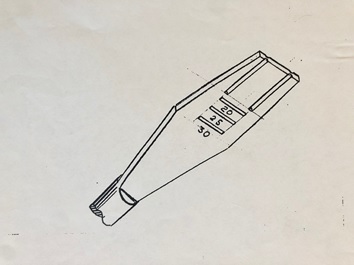
I did not ask for any patent rights or consulting pay because that was not even on my mind. I continued to chase better and more precise ways of approaching spine cases. My natural affinity for craftsmanship and innate appetite for fixing things turned these creations into passion projects rather than chores. As a young adult my hobbies included carving wood, melting metal and creating clay castings.
Next, I tackled development of a titanium cage for anterior fusion through DePuy. In anterior cases at the time, surgeons would place cylindrical, or Ray’s cages, or they would insert structural allograft. The problem is that cylindrical cages cut into the vertebral body, where it would compromise the natural lordosis. The structural allograft, although it worked really well, was cumbersome to cut to the exact height. It is also difficult to source allograft. Therefore, I did not feel that either of those mechanisms were ideal approaches to stabilize the vertebral body. Instead, I designed the titanium cage in an octagon shape with different heights. Filled with morselized bone graft, the cage proved to be a solid and safe alternative. I implanted 90 of these on custom order. I had excellent results for my patients.
Image 2: Sketch of anterior titanium cage
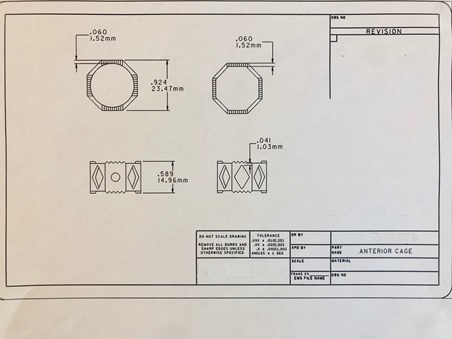
Over the years I had developed my own method of handling spondylilosthesis. To improve surgical performance for these cases, I began the process of developing a reduction screw. I communicated with AcroMed what I had envisioned and they built it, but engrossed in my other ideas, I had no time to lend it the attention it deserved. I did not file a patent for it and later regretted this because the reduction screw became a widespread tool in spondylilosthesis.
At some point in the early 1990s DePuy launched its polyaxial screw. This was a big step forward from solid post screws that carried a multitude of technical problems, particularly for patients whose spines were not properly aligned. Despite the progressive improvement, omission of some fine details made it less than perfect. This polyaxial contained an inner set screw and an out nut. The combination of the two generated enough force to push the spherical head of the screw into a cone-shaped housing, creating a friction lock. If you tightened the set screw too soon, then the nut would not fit in properly. If you tightened the nut, then the set screw would be difficult to get started. In order to tighten it enough so the screw head would friction lock with the housing, there was a lot of rotational force. This was the other issue because this force would flip the rod to the right, making it difficult for the entire construct. Needless to say, there was a lot of tinkering involved.
The constrictions and hazards this tool posed drove me to develop my own polyaxial screw. I wanted to design a system that would be simpler to assemble and to lock, which would effectively decomplicate the surgery and mitigate unnecessary risks. At this point I was well into expanding my own practice, where I was seeing up to 150 patients and operating at least 10 major cases per week. I spent my spare time working with engineers and later draftsmen at their machine shop to come up with this new type of screw.
One after another we worked on finetuning even the slightest details to achieve the right design, and finally, after 18 variations, we created a simple, effective design. A washer that locks onto the screw head had ridges with hardened titanium. In a set screw that would lock the screw head with only 20 pounds of pressure, therefore failing to allow for any lateral rotation. Our details meant the surgeon would actually be able to torque the set screw to 100 pounds without fear of the rod rotating. The next step was taking this prototype from the lab to the outside world. I learned that the next step was to perform proper mechanical testing and have a consultant write up a report to submit to the FDA.
I researched within the industry, gathering information as to what type of titanium was typical for medical use and where we could conduct mechanical testing. A challenging next step was locating a machine shop that had actual experience developing medical devices. Most established spine companies had in-house machine shops, but I was still a one-man operation at the time.
After consultants at the shop made all the screws that were needed, I submitted them for biomechanical testing. Very favorable results in relations to other existing systems in the market returned. I made sure to be extremely thorough and specific, and had my engineers overcalculate everything since the intention was to design the best system for patient and physician use. The result was a paragon of excellence for performance in posterior fixation operations. Soon after, we submitted for FDA approval and were approved the first time with no issues.
I used the polyaxial screw system in my own practice for two years to ensure the outcome was failure-free and favorable. It performed very well. I named it the Haider UCR Spinal Screw System and committed to donating all royalties to the University of California Riverside to aid in developing a medical school.
I then wanted to make the pedicle screw system available to spine surgeons throughout the country, but sometime towards the end of 2000 Kirt Stephenson approached me to license my pedicle screw for AlphaTech. Instead of licensing the screw, I offered him the role of helping me make this product available nationwide. In 2001 I formed SeaSpine Inc. and hired Kirt to serve as the chief executive officer.
Kirt got to work and in that first year brought with him a sales manager and then a vice-president of engineering, an engineering director and a supply chain worker. I designated private office space for the four employees at my clinic, Haider Spine Center. We ran the company very lean but I bankrolled the entire operation, using my own money that I had made at my practice and personally signing a line of credit. This was a huge financial risk. From the beginning I was approached by venture capital firms that wanted to invest in the company, but that was not something I was interested in. This would inevitably shift the focus to a profit mode rather than maintaining the physician and patient-centric approach.
Early on I revised the pedicle screw to make it even less complex by solidifying the cap and the set screw into one unit. It was re-named “Malibu.”
There was also another, minor issue that I wanted to rectify before making Malibu. During a case, screws of various sizes are needed depending on the specific step in the procedure. An inordinate amount of time is often wasted, and concentration disrupted in the operating room when surgical technicians measure each screw on the spot—leaving the surgeon on hold until the correct millimeter is found. When making Malibu, I decided to color code each size so that I could just ask for the pink screw, for example, instead of waiting for a hasty measurement. This began near the winter holidays and we picked bright colors for the screws, and red and green colors for the two sizes of the screw drivers.
Image 3: The Malibu screw in blue

Soon, other companies followed and color coded their items too. I should have filed a patent for this idea as well, but again, simplification and efficiency were on my mind before ownership and monetary gain.
Image 4: Cervical plates of various sizes

We added more engineers during the third year and launched a cervical plate system. Later I led our engineers through the design and development of a cervical cage system as well as posterior lumbar interbody cage and transforaminal lumbar interbody cage systems. We also developed anterior lumbar cages with a plate system. Although I did a lot of the nuts and bolts work myself, a critical component of SeaSpine was involving the perspectives of various physicians. We brought respected surgeons from throughout the country on board as consultants to garner their input and offer them a sense of ownership of the products. The feasibility of our products and emphasis on educating doctors and fostering collaboration attracted many users. Many of the doctors became well acquainted with one another through our educational conferences, and our philanthropic leg that was there from day one continued to evolve. Every year a number of our surgeons would travel to Mexico or the Dominic Republic to perform pro-bono operations on the underserved using SeaSpine technology. These were rewarding and bonding experiences for the doctors and fruitful trips where the doctors would correct multiple deformities, primarily on children, in a place where access to spine care was limited or nonexistent.
Development at the company was going strong and we eventually carried numerous instruments that the doctors required, providing us a competitive edge against other corporations. Kirt was doing an excellent job with administration and maintaining a well-lubricated operation. We continued to expand SeaSpine in San Diego’s north county, eventually opening a second location for a total of 35,000 square feet of office space that included a cadaver lab.
Investment bankers approached us on a regular basis seeking shares in SeaSpine, but I, chairman of the board, was not ready to relinquish control of a company that was doing so well under our watch.
By 2010 our sales were topping $50 million per year. It was at that time that Integra Life Sciences, a publicly traded company, approached us with a purchase offer.
We had attorneys on board, but I handled most of the exchanges and paperwork. As it was, I was already wearing many hats and by that time I trusted my legal skills as I had spent years meticulously reviewing and editing business and patent documents. At the time of purchase, we had over 50 employees and were well on our way to developing a MIS system, a motion preservation system and a deformity system. The intervertebral dynamic system, or IDS, was a brainchild of mine. A dynamic cage system that would facilitate a soft fusion, it does not allow full movement but permits some motion, to act as a shock absorber. The deformity system for scoliosis patients adds a lot of instrumentation that helps doctors correct deformity. Our products that were already in use included two different types of anterior cervical plates, cervical cages, posterior cervical screw fixation including cross link, posterior lumbar pedicle screw fixation with different length of reduction tabs, thoracic hook instrumentation with various reduction tabs, posterior lumbar interbody cages, posterior lumbar oblique cages, transforaminal lumbar interbody cages, anterior lumbar interbody cages with various lordotic angles, anterior lumbar interbody cage combined with plate, anterior lumbar plate fixation and cross link for lumbar fusion. Sometime after the deal was finalized, Integra spun SeaSpine into a publicly traded company that exists today.
The success of SeaSpine had three components. One, my desire to improve tactics in spine surgery to afford more safety for patients and feasibility for doctors. Second, my willingness to take on a large financial risk. I played the biggest gamble of my life by putting all my holdings at stake, but all along I believed in the potential of the company. There is always an idea out there, but manifesting the thought through action is crucial. Third, having an open-door policy and including spine and neurosurgeons from all over to partake in discussions allowed for essential improvement and provided good scientific consensus on the design of the products.
My wife, Salma had introduced a great idea of hosting an annual user’s meeting where all the consulting surgeons of SeaSpine would get together for a long weekend, bring their cases and discuss their experiences with the products. Our engineers engaged and help them modify the products or develop new ones. This was actual one-on-one consulting, and not merely reps walking into an operating room to observe the doctor at work. All these physicians felt a sense of ownership; using their medical knowledge to design something and having SeaSpine as an avenue to improve their own practice and patient care was a win for everyone.
I had vacillated between the pros and cons of selling the company before ultimately deciding that I had done my work. I had accomplished my objectives that stemmed from my early days of surgery in Colorado—improving surgical performance, increasing safety measures for patients under the knife and sharing my ideas and inventions with other surgeons to replicate.
Previous chapters:
Dr. Richard Guyer: Today's spine fellowship
Challenges, risks and opportunities in today's spine world
Spine care - Balancing cost with innovation
What are big data and predictive analytics
Predictive Analytics and Machine Learning
The HSS Spine Care Model, Part 1
The HSS Spine Care Model, Part 2
The History of Texas Back Institute
Private practice vs. hospital employee: Where we are today and why
Episodes of care and bundled payments
Episodes of care and bundled payments, a sustainable approach
Dr. Scott Blumenthal on specialty hospitals
Spine industry trends in new technologies and market challenges