Dayton, Ohio-based NovoSource received FDA clearance for its NovoKnee total knee replacement system, according to the Dayton Business Journal. 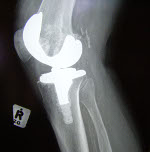 The implant is designed to be a more cost-effective alternative to traditional orthopedic joint replacement technologies, according to the report.
The implant is designed to be a more cost-effective alternative to traditional orthopedic joint replacement technologies, according to the report.
NovoSource was founded in 2012 with monetary support from the Dayton Development Coalition, which is led by Harold Linville, former chairman for Innovative Medical Device Solutions and current NovoSource chairman and CEO. The company is also developing the NovoHip implant to market this year.
More Articles on Devices:
Medtronic Spine Launches Device for Multilevel Spinal Fusion
Wenzel Spine Launches Cervical Interbody Fusion Implant
Spine Wave Intervertebral Body Fusion Device Receives FDA 510(k) Clearance
 The implant is designed to be a more cost-effective alternative to traditional orthopedic joint replacement technologies, according to the report.
The implant is designed to be a more cost-effective alternative to traditional orthopedic joint replacement technologies, according to the report. NovoSource was founded in 2012 with monetary support from the Dayton Development Coalition, which is led by Harold Linville, former chairman for Innovative Medical Device Solutions and current NovoSource chairman and CEO. The company is also developing the NovoHip implant to market this year.
More Articles on Devices:
Medtronic Spine Launches Device for Multilevel Spinal Fusion
Wenzel Spine Launches Cervical Interbody Fusion Implant
Spine Wave Intervertebral Body Fusion Device Receives FDA 510(k) Clearance

